Vapor Pressure Formula
When a liquid evaporates, the gaseous molecules created escape into the air. If the liquid is in a closed container, the gaseous molecules created will not escape but remain above the liquid. These evaporated particles created a pressure above the liquid, this is known as the vapor pressure.
If a solid is dissolved into the liquid, a solution is created. The vapor pressure of the solution is lowered by the addition of the solute. Raoult’s law explains how the vapor pressure of a liquid is altered by the addition of a solute.

Psolution = the vapor pressure of the solution
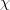 solvent = the mole fraction of the solvent in the solution
solvent = the mole fraction of the solvent in the solution
P°solvent = the vapor pressure of the pure solvent at standard conditions
Vapor Pressure Formula Questions:
1. What is the vapor pressure of a solution at 25°C containing 3.5 moles of glucose in 10.0 moles of water? (the vapor pressure of pure water at 25°C is 23.8 torr)
Answer:
In order to solve for Raoult’s law, the mole fraction of water must be obtained.
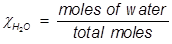
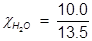
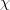 H2O=0.741;
H2O=0.741;
Using the mole fraction of water, solve for Raoult’s law.

Psolution=0.741 x 23.8 torr
Psolution=17.6 torr
2. What is the vapor pressure of a solution at 25°C when 25.5 grams of glucose (C6H12O6) is dissolved in 212 grams of water? (the vapor pressure of pure water at 25°C is 23.8 torr)
Answer:
In order to solve for the mole fraction of water, both the grams of glucose and grams of water must be converted to moles.
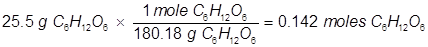
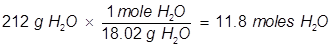
Now solve for the mole fraction of water.
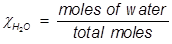
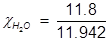
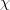 H2O=0.988;
H2O=0.988;
Finally, solve for the partial pressure of the solution.

Psolution=0.988 x 23.8 torr
Psolution=23.5 torr
|
Related Links: Gases: Pressure Quiz Pneumatics Examples Pressure Formula Gay-Lussac's Law Formula Boyle's Law Formula Osmotic Pressure Formula Ideal Gas Law Formula |
